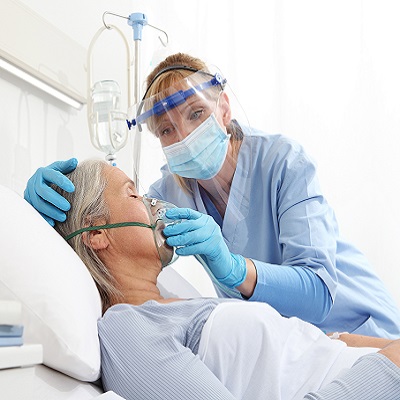
How Custom Workwear Can Support Infection Prevention Protocols in Hospital Environments
Did you know that according to the National Library of Medicine, everyday healthcare apparel is usually contaminated with pathogens and microorganisms? These pathogens can cause different types of diseases ranging from vancomycin-resistant bacteria (VRE) to Clostridioides. vancomycin-resistant bacteria affect the bloodstream and urinary tract system. It can survive for days on dry surfaces, including standard hospital gear. Similarly, Clostridioides

The Future of Long-Term Care: Integrating Pharmaceutical and Technological Advancements
Long-term care (LTC) is at a turning point. As the population ages and chronic health conditions increase, the demand for high-quality healthcare services continues to grow. Traditional care methods are struggling to keep up, but advancements in medical science and technology offer promising solutions.

Telepharmacy and Remote Healthcare Services: Bridging the Gap for Elderly Patients
Elderly patients in remote areas face growing challenges in accessing medications, largely due to pharmacy closures that leave them isolated from essential healthcare services. These closures disrupt adherence to prescriptions and can worsen health outcomes as seniors struggle to obtain refills or consult pharmacists. Telepharmacy and remote healthcare services offer a solution, leveraging technology to deliver medications and medical support directly

Easier-to-use EMR Systems Benefit Clinicians and their Patients
Imagine, like me, that you were a clinician starting their medical career with the UK National Health Service (NHS). You discover a valuable resource that provides instant access to a patient?s medical history and insights into previous diagnoses and treatments ? the electronic medical record (EMR) system.

Make the Most of AI in MedTech with Data Know-How
With artificial intelligence in healthcare, data is not just a minor component; it?s the foundational layer that amplifies AI?s potency, particularly in MedTech. When we peel back the layers of AI, we uncover crucial elements that engage with and depend on data in fundamental ways. A new InterSystems whitepaper explores these relationships making data the bedrock of AI

Livingston Hospital has achieved a significant milestone in its plan to develop a modern facility, supported by a $78 million investment from the U.S....

Memorial Hermann Cypress Hospital has begun work on a $277.5 million campus expansion to support the rapidly increasing health care needs of the Cypre...

Work has begun on the $95 million redevelopment of Temora Hospital, marking an important step towards improving healthcare services for Temora and nea...

Memorial Health has announced plans to build a new, state-of-the-art emergency department (ED) on its hospital campus, with an estimated investment of...
Dental South China 2026
ECR 2026
International Forum on Quality & Safety in Healthcare OSLO 2026
HIMSS Global Health Conference & Exhibition 2026
Medtronic Announces FDA Clearance for Stealth AXiS Surgical System in Spine Procedures
Medtronic, a leading global healthcare technology company, has officially announced the...
Cynosure Lutronic Secures Dual Regulatory Approvals for Clarity II Laser Platform in China...
Cynosure Lutronic, a global leader in energy-based medical aesthetic devices, has achie...
Morristown and Overlook Medical Centers Named Among World's Best Hospitals for 2026 by New...
Atlantic Health System's Morristown Medical Center and Overlook Medical Center have bee...
GSMA Foundry Partners with Singapore's NUHS to Deploy 5G, AI, and XR for Smart Hospital Tr...
The GSMA Foundry, an industry collaboration program, has entered into a landmark strate...
Novartis Announces New Radioligand Therapy Manufacturing Facility in Denton Texas to Enhan...
Novartis a leading global innovative medicines company has announced plans to establish...
Asian Hospital and Medical Center Unveils Fantastic 6 sa 26 Big Idea Strategic Plan for 20...
Asian Hospital and Medical Center (AHMC), a leading healthcare institution in the Phili...
Edwards Lifesciences Advances Structural Heart Care Through Innovation and Purpo...
At the close of Heart Month, Edwards Lifesciences underscored its leadership in struc...
Quantum Surgical Acquires NeuWave Medical, Inc.
Quantum Surgical, a company specializing in minimally invasive robotic-assisted cance...
CereVasc, Inc. Announces Publication of a Multi-Center Study Evaluating the eShu...
CereVasc, Inc., a clinical-stage medical device company developing novel treatments f...
OneMedNet Announces Strategic Neuro Data Partnership with Risorius to Advance EE...
OneMedNet Corporation, a leading provider of regulatory-grade, AI-ready Real-World Da...

A Journey Through Healthcare's Evolution: Reflections and a Vision for Asia
.png)
RAS Overview: Global Situation, Outlook, and Demand
.png)
Transformative Healthcare It Success: Achieving Emram Stage 7 and Innovating Chronic Disease Management

Digital Health Twin: The Final Frontier in Medicine

Marketing Manager
LBN Medical AS

































.jpg)







.png)
-(1).png)




